History of hot-dip galvanizing
- Hot-dip galvanizing originates from Paul Jacques Malouin, a French chemist who described a method of coating iron by dipping it in molten zinc in a lecture before the Royal Academy of France in 1742.
- In 1772, Italian Luigi Galvani discovered the electrochemical process that occurs between needles in an experiment with frog legs. in an experiment with frog legs.
- Alessandro Volta furthered his research on galvanizing when he discovered the electric potential between two metals, creating a corrosion cell. in 1801.
- In 1836, French chemist Stanislas Sorel received a patent for a method of coating zinc with iron, after first cleaning it with 9% sulfuric acid (H2SO4) and melting it with ammonium chloride. (NH4Cl).

What is hot dip galvanizing?
Hot-dip galvanizing is a series of alloys of zinc and iron formed after dipping in molten zinc due to a metallurgical reaction between iron and zinc to create a coating to protect the material from the effects of the environment. Surrounding environment such as marine environment, acidic environment When hot-dip galvanizing the material can limit the possibility of steel being oxidized.
Hot dip galvanizing process
Hot-dip galvanizing involves the following 3 main steps: surface preparation, galvanizing and inspection. The technical standards are based on the worldwide common ASTM standard for plating criteria of iron and steel products.
Step 1: Prepare the surface
- Surface cleaning is the first and most important process in galvanizing. If the surface is not cleaned, the necessary reactions between the steel and zinc surfaces will not take place resulting in discontinuous plating and damaged galvanized products.
- Depending on the dry or wet galvanizing process, the surface preparation process is divided into 2 or 3 steps. Regarding wet hot-dip galvanizing, this includes surface degreasing (old paint peel or other residue) and degreasing. While hot-dip galvanizing is not, we need an additional cleaning step that is dipping the flux bath after surface degreasing and degreasing.
Step 2: Galvanized
- After the surface preparation is completed, the clean composition is transferred to a galvanized kettle and dipped into a bath of molten zinc. The temperature of the molten zinc bath is generally kept at ~450 C, about 30 C higher than the melting point of zinc. Such high temperature of molten zinc allows for greater fluidity and better adhesion to the steel surface. During the soaking process, the molten zinc reacts with the iron on the surface of the material and forms several layers of zinc-iron intermetallic alloy. The outermost layer of the coating is usually composed of pure zinc.
Step 3: Check
- After taking the product and letting it cool. Check for any residual surface zinc and remove it by filing. Care should be taken as this may damage the coating. If other methods are used, international ASTM standards must be met. Shipping galvanized products must be transported inside separate and closed moisture-free containers so that air can circulate between the gaps.

Standard Hot Dip Galvanized
There are currently 3 main standards in hot-dip galvanized steel and a number of other supporting techniques that engineers have specifically designed to promote coatings that ensure steel designs are suitable for hot-dip galvanizing.
ASTM Standard
- ASTM A123/A123M:Standard specification for hot dip galvanized coating on iron & steel products
_ Single pieces of steel or manufactured with different steel grades - ASTM A153/A153M:Standard specification for galvanizing (hot dip) on iron and hardware
_ Small products are centrifuged after galvanizing to remove excess zinc - ASTM A767/A767M: Standard specification for galvanized (galvanized) steel bar for concrete reinforcement
_ Rebar
Other galvanizing standards
- CSA G164:Hot-dip galvanizing for irregular shaped items
- ISO 1461:Hot dipped galvanized coating on pre-engineered steel buildings
- AASHTO M111 ( ASTM A123):Zinc coating (hot dip galvanizing) on iron and steel products
- AASHTO M232 ( ASTM A153): Galvanized coating on high hardness steel
Performance
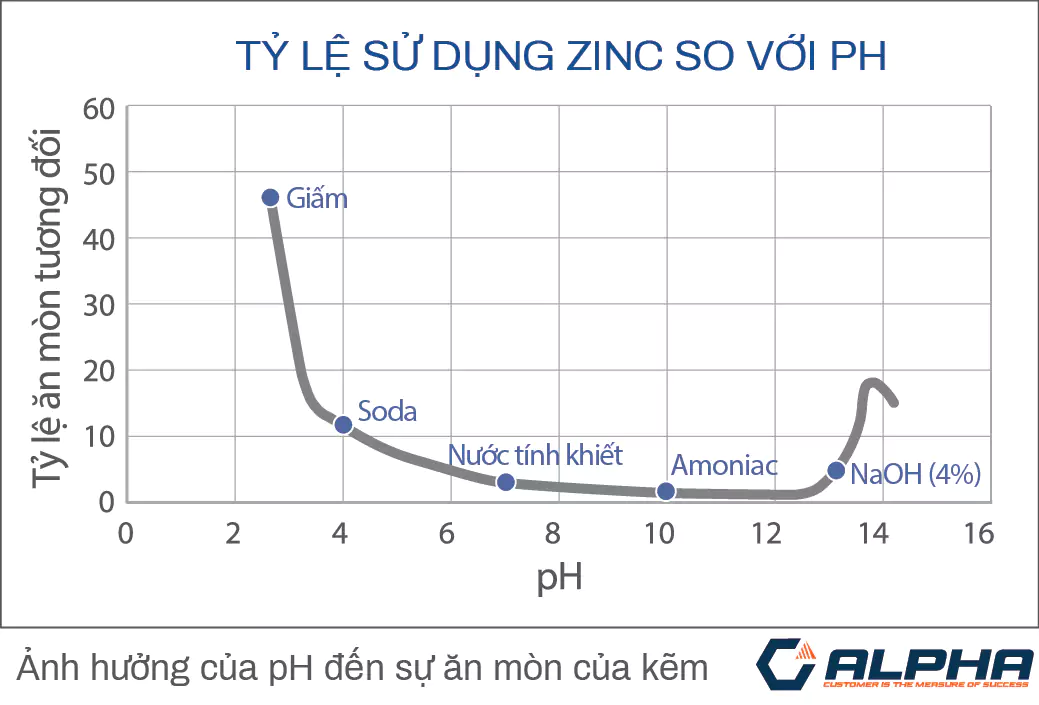
The performance of galvanized coatings is proven under a variety of environmental conditions. The corrosion resistance of the coating is determined mainly by the thickness and depending on the change of environmental conditions
Environments where hot-dip galvanizing is often required include outdoors, chemical storage, fresh water, seawater or in concrete. Because zinc plating can resist corrosion for many years. Normally galvanizing will work well in solutions with pH above 4.0 and 12.5.(See picture below)